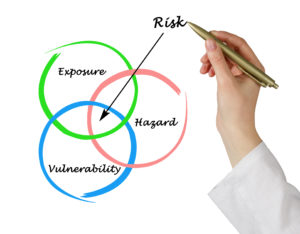
Hazard Analysis Program
Environmental Testing


Hazard Analysis Program
First Priority maintains a robust hazard analysis program that reviews the potential for risks associated with each manufacturer of ingredients following NSF guidelines.
First, four members of our quality team review the quality program of a potential vendor, utilizing physical audits, interviews, testing method validations, and background checks for past or current regulatory issues.
Next, all compiled information is brought to a classification session. During this session, a review of the documents and ingredients supplied by the potential vendor is conducted. Potential hazards are proposed such as country of origin, inherent ingredient hazards (mold, E. coli, Salmonella…) or economical adulteration potentials.
After all potential hazards have been agreed upon and classified, the probability of such hazards occurring is determined, using a classification from frequent (1 in 5 purchases) all the way down to extremely unlikely ( 1 in 10,000 purchases). These are determined by scientific methods, observations, real-life testing protocols, and industry inside knowledge of economically generated adulterations. Last, using the severity and probability, a risk level is determined.
Environmental Testing
Environmental testing for microbiological bioburden hazards is an integral part of the testing program designed by First Priority Manufacturing.
Introduction of microbial adulterants during holding or processing is monitored utilizing USP Method 1116 for continued evaluation of microbial limits in controlled areas. This method was adopted by First Priority Manufacturing voluntarily, from the pharmaceutical codes. Dietary supplement regulations do not require this, but we believe it is imperative for quality.

Organic and Whole herbs are subjected to irradiating, and ETO(a pesticide) for sterilization in many cases. Even though irradiation is not approved by the United States and the European Union, it still is prevalent, especially in “organic “ materials due to their naturally high microbial contents.

First Priority Manufacturing guarantees that all our botanical’s are radiation-free. Unlike many of our competitors, we have a standard monitoring program to insure that no radiation is used in the sterilization of our botanical’s. When sterilization is needed, we use a dry-steam technology or other non-destructive technologies. (Note: only whole unprocessed herbs require treatment, and only when their total aerobic counts are found to be out of compliance.)
We use our international sources to achieve the ultimate benefit of natively grown plant sustainably cultivated in its natural habitat. This has lessened the use of pesticides or fertilizers. While protecting endangered herbs.
Every day worldwide 2500 acres of raw land are disturbed by man. According to GSA Today as of 1995, ~43% of Earth’s surface area had experienced human-induced degradation (Daily, 1995). Ellis and Ramankutty (2008) concluded that more than 75% of Earth’s ice-free land area could no longer be considered wild. Of earth’s ice-free land area, 83% is likely directly influenced by human beings (Sanderson et al., 2002). Our pollutants affect plant and animal physiology worldwide (McKibben, 1989, e.g., p. 38, 58)
Needless to say the world’s gene pool of wild herbs is dwindling. By searching out strong stable international connections Priority One maintains eco friendly sources for our formulations. Lending our support to companies that are good stewards of the earth, such as Naturex who has a proven record of sustainability-program-principles.
We have developed an industrial networking relationship with international companies who have developed unique complex systems for animal health, environmentally responsible botanical harvesting, and 100% radiation free sterilization techniques.
70% of ALL antibiotics sold in the United States are consumed by HEALTHY animals. 56% of Australian antibiotics are consumed by their healthy animals, and while not publishing the percentage of antibiotic use for their healthy animals New Zealand’s Foodsafety.govt.nz supports the needed continued use of antibiotics for “preventative or prophylactic use”
90% of our our glands are sourced from Denmark, and unlike Australian, or New Zealand glands, Denmark is the only country that has been antibiotic, and growth hormone free for all bovine and and porcine glands since 1998. This means that our glands are pure, untreated, and retain the best balance of naturally occurring hormones available on the market. The last 10% are sourced from New Zealand.
Floods, droughts, fires, and earthquakes are major causes of adulteration in the food chain.
 Through our Hazard Analysis Program we are able to block natural event adulteration’s. All raw ingredients have potential risk of contamination, bioburden, and other dangers. By identification of such risks ahead of time, the mitigation process enables us to significantly reduce such hazards. First Priority has developed a robust Hazard Analysis Program that does just that.
Through our Hazard Analysis Program we are able to block natural event adulteration’s. All raw ingredients have potential risk of contamination, bioburden, and other dangers. By identification of such risks ahead of time, the mitigation process enables us to significantly reduce such hazards. First Priority has developed a robust Hazard Analysis Program that does just that.
 Our Quality Assurance team use this program to identify hazards in the ingredient supply chain and then develop testing protocols unique to each ingredient. These mitigation’s create targets for testing. Designing a minimum of 1 upstream and one downstream precaution for each hazard that is identified. Hazard Analysis Program provides more information on how we block adulteration’s.
Our Quality Assurance team use this program to identify hazards in the ingredient supply chain and then develop testing protocols unique to each ingredient. These mitigation’s create targets for testing. Designing a minimum of 1 upstream and one downstream precaution for each hazard that is identified. Hazard Analysis Program provides more information on how we block adulteration’s.
We do not rely on COA (Certificate of Analysis) from raw ingredient manufacturers. We test all raw ingredients for purity, potency, and well as environmental or bioburdens that may be present before we allow any raw materials be used in our manufacturing process. All ingredients are quarantined until they have been approved and released for production.
We realize our integrity in this regard is relied upon by yourself and your brands reputation. With this in mind we are meticulous in our procuring, testing, verifying and producing the highest quality and clinical strength supplements, that you can stand behind with confidence.
